Statistical controversies in clinical research: limitations of open-label studies assessing antiangiogenic therapies with regard to evaluation of vascular adverse drug events—a meta-analysis - Annals of Oncology

Clinical impact and quality of randomized controlled trials involving interventions evaluating artificial intelligence prediction tools: a systematic review | npj Digital Medicine
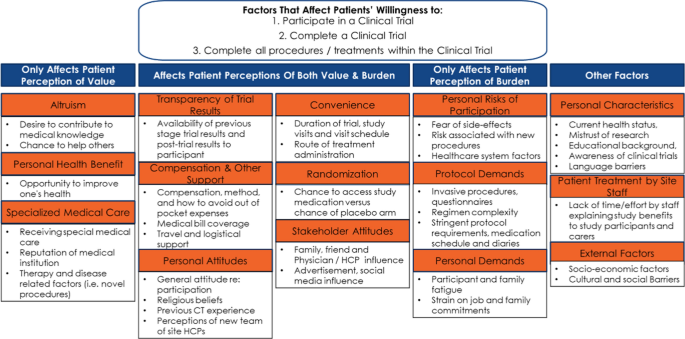
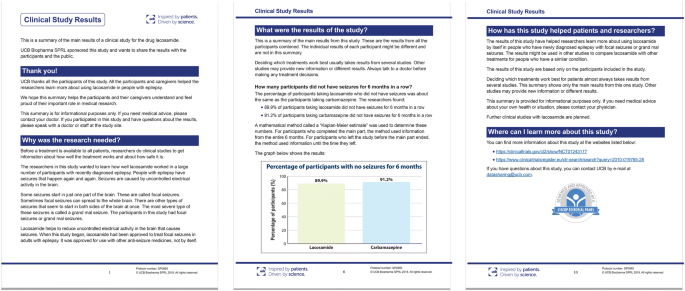
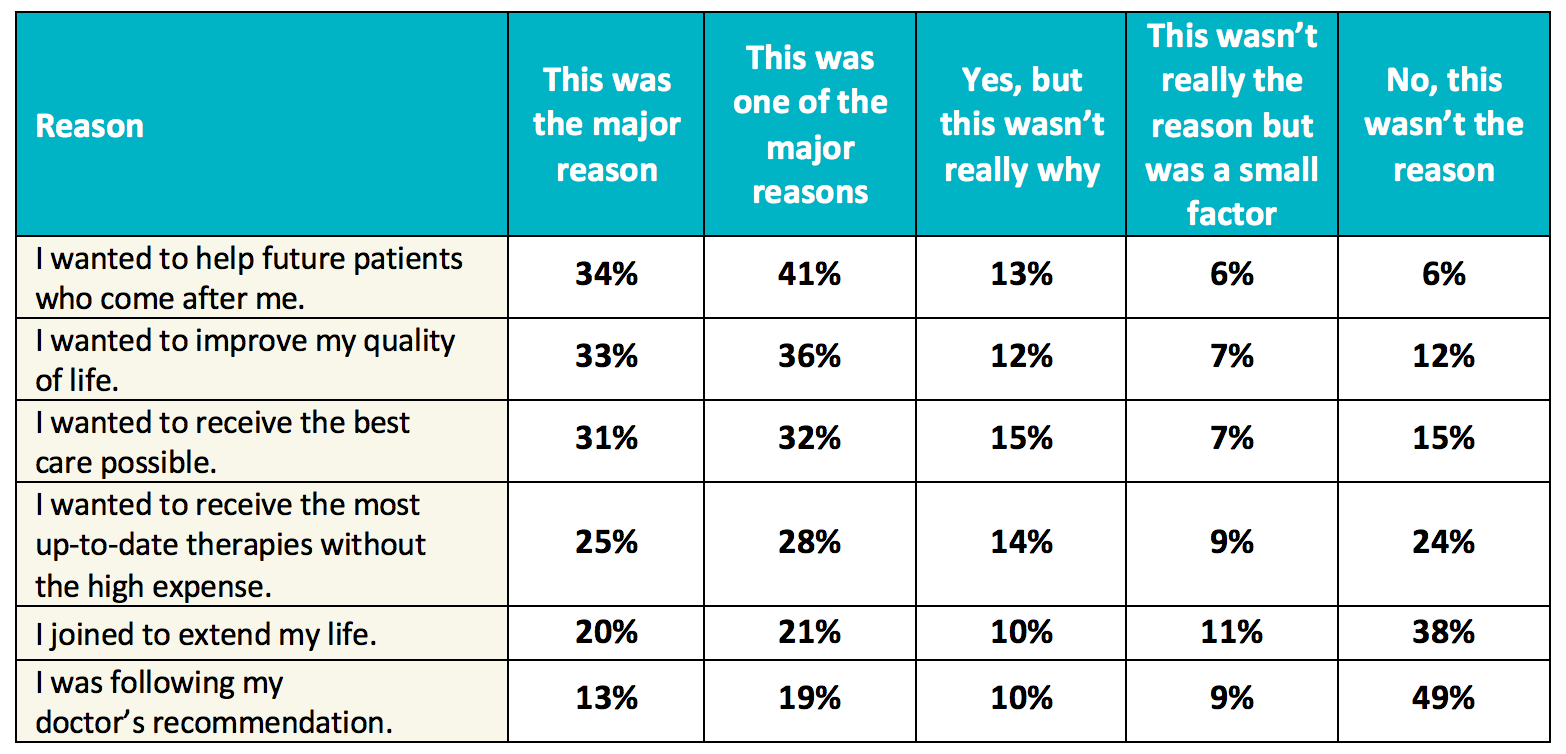
Amplifying the Voice of the Patient in Clinical Research: Development of Toolkits for Use in Designing and Conducting Patient-Centered Clinical Studies | SpringerLink

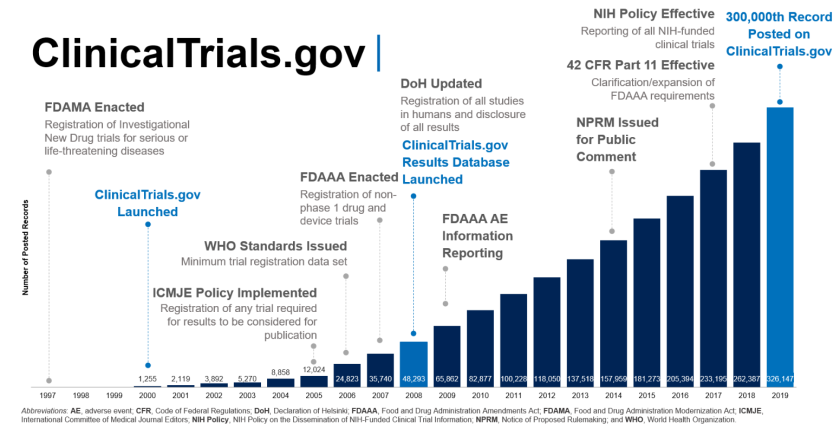
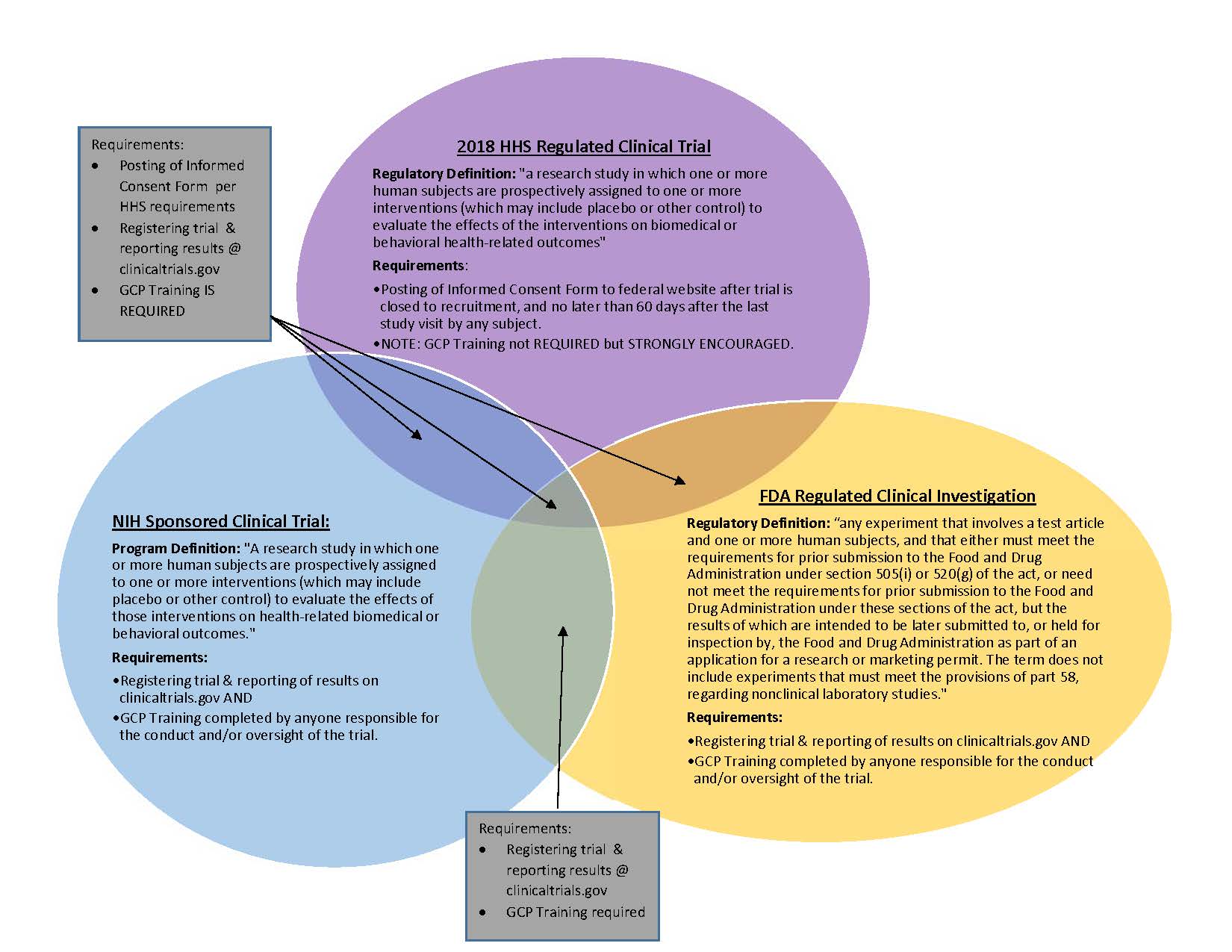
Final Rule Confirms, Posting of Study Results on ClinicalTrials.gov Will be Required for Unapproved Products - IMPACT Pharmaceutical Services, Inc.
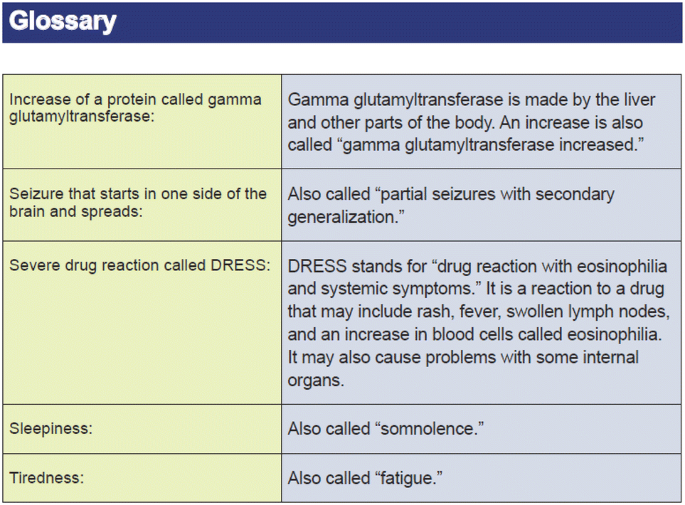
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine
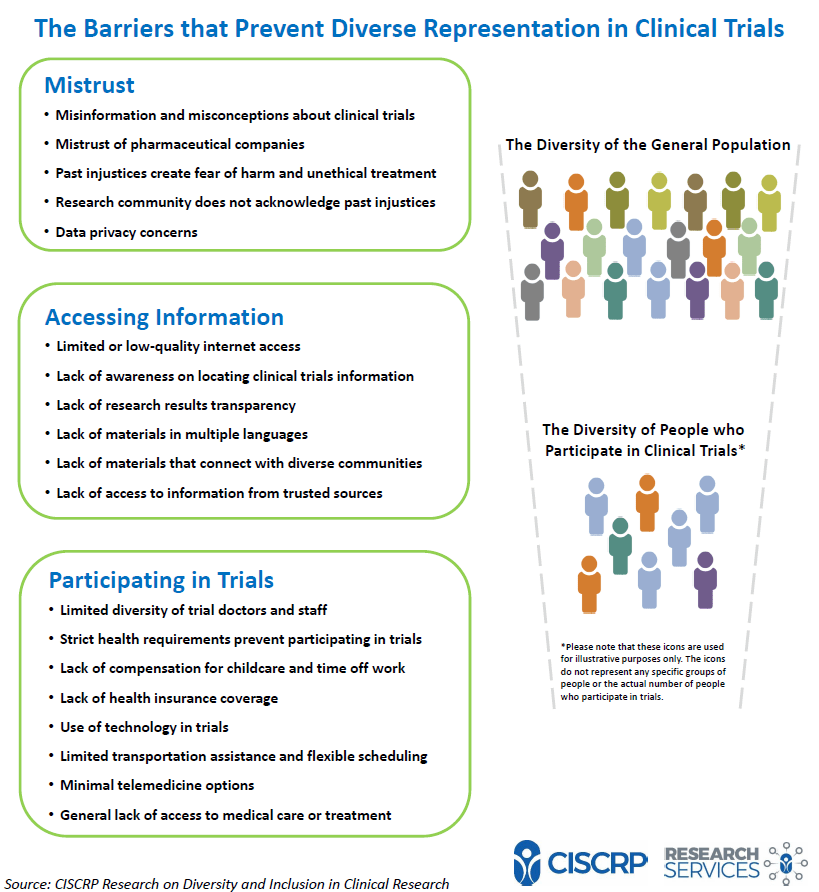
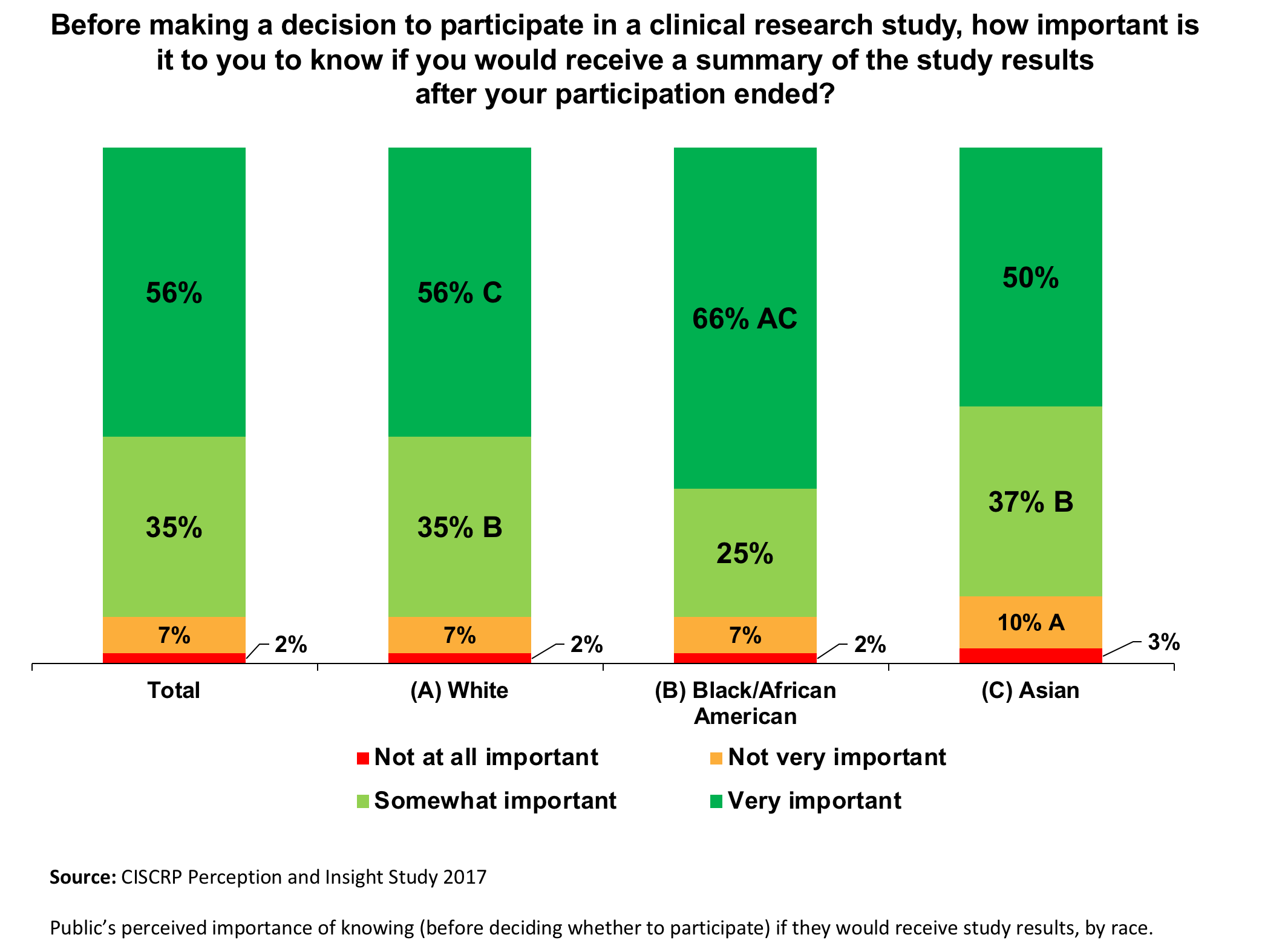
Doing Our Part: Improving Diversity in Clinical Research Participation - Center for Information & Study on Clinical Research Participation
Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials | PLOS Medicine